
Recalls
On this page you’ll find recent recall alerts for food and feed products distributed or produced in Georgia. These alerts include the reason for the recall, a description of the issue, and a complete listing of affected products with identifying information.
Learn more about recallsGooder Foods, Inc. (09/08/2025)
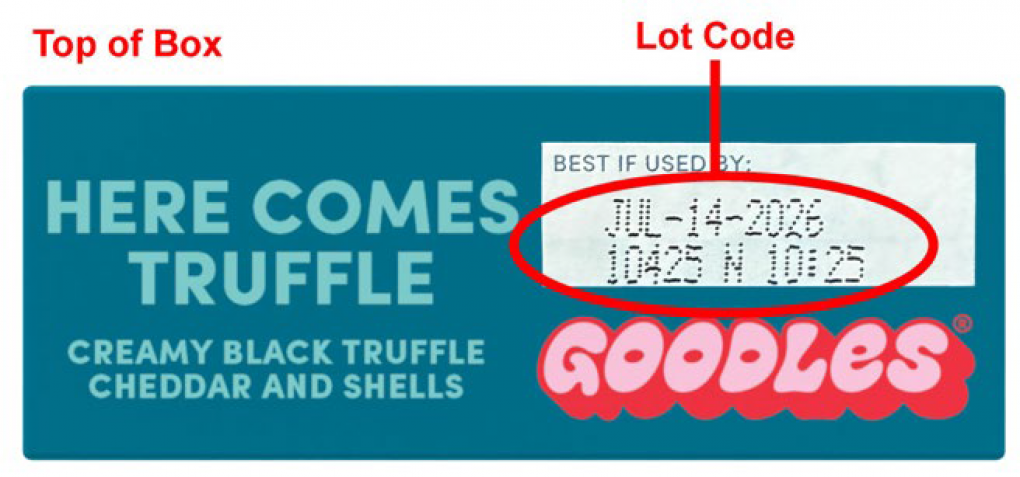
Gooder Foods, Inc. is recalling 5 lots of Vegan Is Believin’ – Plant Based White Cheddar with Spirals produced as they may contain milk, which is not listed on the label, and 3 lots of Here Comes Truffle – Creamy Truffle Flavored Cheddar and Shells, as they may contain cashew which is not listed on the label. The recalled lots were produced between April 7 and April 15, 2025.
Persons who have an allergy or severe sensitivity to milk and/or cashew may run the risk of a serious or life-threatening allergic reaction if they consume these products.
| Product | Lot Code | Best Buy Date | UPC | Packaging |
|---|---|---|---|---|
| Goodles Vegan is Believin’ – Plant Based White Cheddar with Spirals |
09725N 09825N 09925N 10025N 10125N |
Jul-7-2026 Jul-8-2026 Jul-9-2026 Jul-10-2026 Jul-11-2026 |
850031990074 | 5.25 oz |
| Goodles Here Comes Truffle – Creamy Truffle Flavored Cheddar and Shells |
10125 N 10425 N 10525 N |
Jul-11-2026 Jul-14-2026 Jul-15-2026 |
850031990159 | 6 oz |
The products were distributed nationally to wholesale distributors and operators and online between 04/29/2025 and 08/05/2025. The applicable UPC and lot codes can be found on the outside of the product, as shown in the pictures.
The issue was discovered through consumer feedback . The company is recalling the affected product and notifying all distributors, retailers, and consumers who may have received the impacted lots.
There have been six reported allergic reactions in connection with Here Comes Truffle and two reported allergic reactions in connection with Vegan is Believin’. Anyone concerned about an allergic reaction should contact a healthcare provider.
Consumers who have purchased the recalled product should not consume it and can return it to the place of purchase for a refund. Customer services representatives are available Monday through Friday from 8am to 8pm EST at 1-888-610-2341.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.





AquaStar (USA) Corp (09/01/2025)
AquaStar (USA) Corp of Seattle, WA is recalling approximately 26,460 packages of Cocktail Shrimp 6oz (see photo), imported from Indonesia, because they may have been prepared, packed, or held under insanitary conditions whereby they may have become contaminated with cesium-137 (Cs-137).
Cs-137 is a man-made radioisotope of cesium. Traces of Cs-137 are widespread and can be present in the environment at background levels, and at higher levels in water or foods grown, raised, or produced in areas with environmental contamination. The primary health effect of concern following longer term, repeated low dose exposure (e.g., through consumption of contaminated food or water over time) is an elevated risk of cancer, resulting from damage to DNA within living cells of the body.
The affected Cocktail Shrimp 6oz was sold only in Walmart stores in AK, AL, AR, CO, GA, IA, ID, IL, IN, KS, KY, LA, MI, MN, MO, MS, MT, ND, NE, OH, OK, OR, SD, TN, TX, WA, and WI between July 31, 2025 and August 16, 2025. The product was sold in refrigerated condition and has a 12-day shelf life and with various Best if Use By dates.
The affected Cocktail Shrimp 6oz is packaged in a clear plastic tray and has a red and white label. The recalled product has the UPC 19434612191 and the Lot Codes 10662 5106, 10662 5107, 10662 5124, and 10662 5125 at the bottom of plastic tray.
No illnesses have been reported to date.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.
Consumers who have purchased affected shrimps should not consume the product and should dispose of or return it to the place of purchase for a full refund.
Consumers with questions may contact AquaStar at 1-800-331-3440 Monday-Friday, 8am-5 pm PST.

Blue Bell Ice Cream (08/25/2025)
Blue Bell Ice Cream is voluntarily recalling a limited quantity of Moo-llennium Crunch Ice Cream half gallon packaged in a Chocolate Chip Cookie Dough carton produced in its Brenham, Texas, plant because of undeclared almond, walnut, and pecan. The recalled product was mistakenly packaged in Chocolate Chip Cookie Dough ice cream cartons with a Moo-llennium Crunch lid. People who have an allergy or severe sensitivity to almonds, walnuts, and pecans run the risk of a serious or life-threatening allergic reaction if they consume these products.
A Blue Bell employee discovered the incorrect packaging on two half gallons while restocking a retailer. No illnesses or adverse reactions have been reported to date. No other incorrect packaging has been discovered or reported to date.
The half gallons can be identified as Moo-llennium Crunch Ice Cream packaged in a Chocolate Chip Cookie Dough half gallon carton with a Moo-llennium Crunch lid and with the following code located on the top of the half gallon lid: 061027524. An image of the affected product is included below.
The affected ice cream half gallons were distributed through retail outlets in Alabama, Arkansas, Florida Panhandle, Northwest Georgia, Southern Indiana, Southern Illinois, Kansas, Kentucky, Louisiana, Mississippi, Missouri, New Mexico, Oklahoma, Tennessee, Texas, and Southwest Virginia.
Consumers who have purchased these items can return them to the place of purchase for a full refund.
For more information, consumers may call 979-836-7977, Monday – Friday 8 a.m. – 5 p.m. CST or email us at consumerrelations@bluebell.com.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.

AquaStar (USA) Corp (08/25/2025)
AquaStar (USA) Corp of Seattle, WA is recalling approximately 18,000 bags (net wt. 2lbs) of Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp because they may have been prepared, packed, or held under insanitary conditions whereby they may have become contaminated with cesium-137 (Cs-137).
Cs-137 is a man-made radioisotope of cesium. Traces of Cs-137 are widespread and can be present in the environment at background levels, and at higher levels in water or foods grown, raised, or produced in areas with environmental contamination. The primary health effect of concern following longer term, repeated low dose exposure (e.g., through consumption of contaminated food or water over time) is an elevated risk of cancer, resulting from damage to DNA within living cells of the body.
The affected shrimp was sold at Baker’s, Gerbes, Jay C, Kroger, Mariano’s, Metro Market, Pay Less Supermarkets, and Pick ‘n Save in AL, AR, GA, IL, IN, KS, KY, MI, MO, MS, NE, OH, SC, TN, VA, WI, WV between July 24, 2025 and August 11, 2025.
The recalled Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp, net wt. 2lbs., is packaged in clear plastic bag and has a white label with green stripes on top of each bag and has the following codes:
- UPC 011110626196, Lot code 10662 5139, Best Before 11/19/2027
- UPC 011110626196, Lot code 10662 5140, Best Before 11/20/2027
The FDA is actively investigating reports of Cesium-137 (Cs-137) contamination in shipping containers and frozen shrimp products processed by PT. Bahari Makmur Sejati (doing business as BMS Foods) of Indonesia. No illnesses have been reported to date. As noted in the FDA statement issued on 8/19/25: “At this time, no product that has tested positive or alerted for Cesium-137 (Cs-137) has entered the U.S. commerce.”
“FDA is working with distributors and retailers that received product from PT. Bahari Makmur Sejati after the date of first detection of Cs-137 by Customs & Border Protection (CBP), but from shipments that did not alert for Cs-137, to recommend that firms conduct a recall. In conjunction with other information, FDA determined that product from PT. Bahari Makmur Sejati violates the Federal Food, Drug, & Cosmetic Act in that it appears to have been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with Cs-137 and may pose a safety concern.”
Consumers who have purchased affected shrimps should not consume the product and should dispose of or return it to the place of purchase for a full refund.
Consumers with questions may contact the company at 1-800-331-3440, Monday-Friday, 8am-5 pm PST.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.

Sabrositos Hondurenos, LLC (08/25/2025)
Sabrositos Hondurenos, LLC, in Edison, N.J., is recalling approximately 32,000 pounds of various meat products that were produced without the benefit of federal inspection bearing labels with a false USDA mark of inspection, the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced today. As more information becomes available, FSIS may update the product list and labels with additional items. Any product bearing the false establishment number “Est. 1785” should be considered misbranded and unsafe to eat.
The meat products, including chorizo (sausage), pork chops, and ribs, were produced on various dates prior to August 20, 2025. The following products are subject to recall [view labels]:
- 14-oz. vacuum-sealed packages containing homestyle chorizo labeled “OLANCHO Chorizo Suelto Olanchano SABROCITOS HONDUREÑOS.”
- 14-oz. vacuum-sealed packages containing smoked pork chops labeled “OLANCHO Chuleta Ahumada Olanchana SABROCITOS HONDUREÑOS.”
- 14-oz. vacuum-sealed packages containing smoked chorizo labeled “OLANCHO Chorizo Ahumado Olanchano SABROCITOS HONDUREÑOS.”
- 14-oz. vacuum-sealed packages containing cased homestyle chorizo, labeled “OLANCHO Chorizo Olanchano Criollo SABROCITOS HONDUREÑOS.”
- 14-oz. vacuum-sealed packages containing smoked BBQ spicy chorizo labeled “OLANCHO Chorizo Parrillero SABROCITOS HONDUREÑOS.”
- 14-oz. vacuum-sealed packages containing smoked ribs labeled “OLANCHO Costilla Ahumada Olanchana SABROCITOS HONDUREÑOS.”
The products subject to recall bear false marks of inspection with establishment number "EST. 1785," which does not exist. These items were shipped to retail locations and restaurants nationwide.
The problem was discovered during routine FSIS surveillance activities when FSIS investigators observed various meat products in commerce bearing false marks of federal inspection.
There have been no confirmed reports of adverse reactions due to consumption of these products. Anyone concerned about a reaction should contact a healthcare provider.
Food produced without inspection may contain undeclared allergens, harmful bacteria, or other contaminants that put consumer health and safety at risk. FSIS is concerned that some products may be in consumer and restaurant refrigerators or freezers. Consumers who have purchased these products are urged not to consume them and restaurants are urged not to serve them. These products should be thrown away or returned to the place of purchase.
FSIS routinely conducts recall effectiveness checks to verify recalling firms notify their customers of the recall and that steps are taken to make certain that the product is no longer available to consumers. When available, the retail distribution list(s) will be posted on the FSIS website at www.fsis.usda.gov/recalls.
Consumers and members of the media with questions about the recall can contact Diego Funez Garrido, Owner, Sabrositos Hondurenos, LLC, at 908-274-4206.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/.





Taylor Fresh Foods (08/25/2025)
In response to a recall initiated by Latitude 36 Foods, LLC., Taylor Fresh Foods is voluntarily recalling the Taylor Farms Honey Balsamic Salad Kit 6/8.3oz. because it may contain undeclared sesame and soy allergens. People who have an allergy or severe sensitivity to sesame and soy run the risk of serious or life-threatening allergic reaction if they consume these products.
Master packs — individual packets of dressing and toppings supplied by Latitude 36 Foods and included in Taylor Farms salad kits — incorrectly included Asian Sesame Ginger dressing rather than the intended Honey Balsamic Vinaigrette dressing, leading to the possibility of undeclared sesame and soy allergens in some Taylor Farms Honey Balsamic Salad Kits.
The Taylor Farms Honey Balsamic Salad Kit 6/8.3oz in scope of this recall was distributed in AL, AZ, CA, CO, DE, FL, GA, IN, KS, KY, LA, MI, MO, MS, NJ, NY, OH, OR, PA, TN, TX, UT, VA, WA and WV and has code dates starting with “TFRS” and “Best If Used By” date up to and including September 4, 2025. The product code can be found in the upper right-hand corner of the packaging.
Consumers who have the recalled salad kit should discard it immediately and not consume it. Refunds are available at the location of purchase. There have been no illnesses reported to Taylor Farms in connection with the recalled product. This recall does not apply to any other Taylor Farms products or brands. Consumers with any questions may contact our customer care team at 855-455-0098 Monday through Friday from 8am-5pm PT.

Beaver Street Fisheries, LLC (08/25/2025)
Beaver Street Fisheries, LLC of Jacksonville, FL is voluntarily recalling a limited quantity of Great Value Frozen Raw Shrimp EZ-Peel & Deveined Tail-On 21-25 Per lb as a precautionary measure per the recommended action stated in an FDA advisory statement dated 08/19/25 regarding Cesium-137 (Cs-137). Cs-137 is a man-made radioisotope of cesium. Traces of Cs-137 are widespread and can be present in the environment at background levels, and at higher levels in water or foods grown, raised, or produced in areas with environmental contamination. The primary health effect of concern following longer term, repeated low dose exposure (e.g., through consumption of contaminated food or water over time) is an elevated risk of cancer, resulting from damage to DNA within living cells of the body.
The product was distributed in the states of AL, AR, FL, GA, KY, LA, MO, MS, OH, OK, PA, TX, and WV, and was available for consumer purchase in select Walmart retail stores in those states from 07/28/2025 to 08/07/2025.
No illnesses have been reported to date.
As noted in the FDA statement issued on 8/19/25: “At this time, no product that has tested positive or alerted for Cesium-137 (Cs-137) has entered the U.S. commerce. FDA is working with distributors and retailers that received product from PT. Bahari Makmur Sejati after the date of first detection of Cs-137 by Customs & Border Protection (CBP), but from shipments that did not alert for Cs-137, to recommend that firms conduct a recall. In conjunction with other information, FDA determined that product from PT. Bahari Makmur Sejati violates the Federal Food, Drug, & Cosmetic Act in that it appears to have been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with Cs-137 and may pose a safety concern.”
Products Affected:
- Product Name: Great Value Frozen Raw Shrimp EZ-Peel & Deveined Tail-On 21-25 Per lb
- Packaging: Plastic bag
- Item UPC / Lot Codes / Best By Dates:
- UPC 078742133898, Lot Code 8005540-1, Best by Date: 3/15/2027.
- UPC 078742133898, Lot Code 8005538-1, Best by Date: 3/15/2027.
- UPC 078742133898, Lot Code 8005539-1, Best by Date: 3/15/2027.
Consumers who have purchased the recalled frozen shrimp should not consume the product and should dispose of the product or return it to the place of purchase for a full refund.
For additional questions please reach out to customer service by phone +1-904-354-8533.
Customer service hours are Monday – Friday, 8AM to 5PM EST.
Link to FDA Safety Alert

FDA Advises Public Not to Eat, Sell, or Serve Certain Imported Frozen Shrimp from an Indonesian Firm (08/18/2025)
Product and stores affected
Certain raw frozen shrimp products processed by PT. Bahari Makmur Sejati (doing business as BMS Foods), a company located in Indonesia, and sold at Walmart.
These products include the following product names, lot codes, and best by dates:
- Great Value brand frozen raw shrimp, lot code: 8005540-1, Best by Date: 3/15/2027
- Great Value brand frozen raw shrimp, lot code: 8005538-1, Best by Date: 3/15/2027
- Great Value brand frozen raw shrimp, lot code: 8005539-1, Best by Date: 3/15/2027
Additional product information is in a table below.
At this time, no product that has tested positive or alerted for Cesium-137 (Cs-137) has entered the U.S. commerce. FDA is working with distributors and retailers that received product from PT. Bahari Makmur Sejati after the date of first detection of Cs-137 by Customs & Border Protection (CBP), but from shipments that did not alert for Cs-137, to recommend that firms conduct a recall. In conjunction with other information, FDA determined that product from PT. Bahari Makmur Sejati violates the Federal Food, Drug, & Cosmetic Act in that it appears to have been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with Cs-137 and may pose a safety concern.
FDA has also added PT. Bahari Makmur Sejati to a new import alert for chemical contamination to stop products from this firm from coming into the U.S. until the firm has resolved the conditions that gave rise to the appearance of the violation.
FDA’s investigation is ongoing. This advisory will be updated as more information becomes available.
Health impacts of cesium exposure
FDA detected Cs-137 in a single shipment of imported frozen shrimp from PT. Bahari Makmur Sejati that did not enter U.S. commerce. The level of Cs-137 detected in the detained shipment was approximately 68 Bq/kg, which is below FDA’s Derived Intervention Level for Cs-137 of 1200 Bq/kg. At this level, the product would not pose an acute hazard to consumers. Avoiding products like the shipment FDA tested with similar levels of Cs-137 is a measure intended to reduce exposure to low-level radiation that could have health impacts with continued exposure over a long period of time.
The primary health effect of concern following longer term, repeated low dose exposure (e.g., through consumption of contaminated food or water over time) is an elevated risk of cancer, resulting from damage to DNA within living cells of the body. Additional information about Cs-137 and your health is available through the Centers for Disease Control and Prevention’s Agency for Toxic Substances and Disease Registry resources.
Recommendation
- If you recently purchased one of the impacted lots of Great Value raw frozen shrimp from Walmart, throw it away. Do not eat or serve this product.
- Distributors and retailers should dispose of this product and should not sell or serve this product.
- If you suspect you have been exposed to elevated levels of cesium, talk to your healthcare provider.
Current Update
August 19, 2025
FDA is actively investigating reports of Cesium-137 (Cs-137) contamination in shipping containers and frozen shrimp products processed by PT. Bahari Makmur Sejati (doing business as BMS Foods) of Indonesia. The U.S. Customs & Border Protection (CBP) alerted FDA to the detection of Cs-137 in shipping containers at four U.S. ports (Los Angeles, Houston, Savannah, and Miami). FDA collected multiple samples for radionuclide analysis, with results confirming the presence of Cs-137 in one sample of breaded shrimp. All containers and product testing positive or alerting for Cs-137 have been denied entry into the country. The agency continues to coordinate with CBP to prevent any contaminated products from reaching consumers and is working with Indonesian seafood regulatory authorities to investigate the root cause of the contamination.
Although testing to date has not confirmed the presence of contamination in any product in commerce, the product appears to have been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with Cs-137 and may pose a safety concern. To date, FDA has learned that Walmart has received implicated raw frozen shrimp, imported after the date of first detection of Cs-137 by CBP, but from shipments that did not alert for Cs-137. FDA has recommended Walmart recall this product.
Consumers should not eat or serve certain lots of Great Value raw frozen shrimp from Walmart:
- Great Value brand frozen raw shrimp, lot code: 8005540-1, Best by Date: 3/15/2027
- Great Value brand frozen raw shrimp, lot code: 8005538-1, Best by Date: 3/15/2027
- Great Value brand frozen raw shrimp, lot code: 8005539-1, Best by Date: 3/15/2027.
If you have recently purchased raw frozen shrimp from Walmart that matches this description, throw it away. Do not eat or serve this product.
Cs-137 is a radioisotope of cesium that is man-made through nuclear reactions and because it is widespread worldwide, trace amounts of Cs-137 can be found in the environment, including soil, food, and air. FDA food monitoring focuses on radioisotopes (radionuclides) that are not normally present and are generally the result of human activities. Any unexpected finding of Cs-137 in a food product is evaluated to determine if follow up action is warranted on a case-by-case basis. After being alerted to the contamination of shipping containers detected by CBP, FDA initiated sampling of products which included five different shrimp products from PT. Bahari Makmur Sejati, one of which was a sample of frozen breaded shrimp. FDA's laboratory confirmation of Cs-137 in the breaded shrimp had detectable levels of Cs-137 present at 68.48 Bq/kg +/- 8.25 Bq/kg. There was no detectable Cs-137 in the other products tested; however, this does not rule out contamination.
FDA has not detected Cs-137 in any product above the current derived intervention levels for Cs-137 (1200 Bq/kg); however, FDA has concluded that the level detected in the breaded shrimp sample could represent a potential health concern for those exposed to this level of Cs-137 from consumption of the shrimp over an extended period of time combined with radiation that exists in the environment and from other sources such as medical procedures. Avoiding products like the shipment FDA tested with similar levels of Cs-137 is a measure intended to reduce exposure to low-level radiation that could have health impacts with continued exposure over a long period of time. FDA has taken swift action to prevent potentially contaminated product from being introduced into U.S. commerce in response to reports of contaminated shipping containers from CBP. On August 14, 2025, FDA posted a new import alert (IA 99-51) for chemical contamination under section 402(a)(4) of the Federal Food, Drug, and Cosmetic Act. PT. Bahari Makmur Sejati has been added to the red list of this alert due to the presence of Cs-137. The import alert ensures that no implicated shrimp products will enter U.S. commerce until the company resolves the conditions that gave rise to the appearance of the violation.
FDA will continue working with industry to trace all implicated products processed by PT. Bahari Makmur Sejati through the supply chain to gather as much information about them as possible and take action as appropriate. Product information will be added to this advisory as it becomes available.
Product Descriptions
| Brand | Product Name | Product Type | Best By | Item Code | Lot Code | Distributor |
|---|---|---|---|---|---|---|
| Great Value | Frozen Raw White Vannamei Shrimp | Frozen Raw Ez Peel Tail-On Farm-Raised White Vannamei Shrimp, 2lb bag | 3/15/2027 | 7383108 | 8005540-1 | Walmart |
| Great Value | Frozen Raw White Vannamei Shrimp | Frozen Raw Ez Peel Tail-On Farm-Raised White Vannamei Shrimp, 2lb bag | 3/15/2027 | 7383108 | 8005538-1 | Walmart |
| Great Value | Frozen Raw White Vannamei Shrimp | Frozen Raw Ez Peel Tail-On Farm-Raised White Vannamei Shrimp, 2lb bag | 3/15/2027 | 7383108 | 8005539-1 | Walmart |
Useful Links
Dollar General Corporation (08/11/2025)
Dollar General Corporation is recalling three (3) lots of its eight (8) ounce Clover Valley® Instant Coffee due to the potential presence of glass.
8-Ounce Clover Valley® Instant Coffee
Package UPC: 876941004069
Lot: L-5163 / Best By 12/13/2026
Lot: L-5164 / Best by 12/13/2026
Lot: L-5165 / Best by 12/14/2026
Customers can find the lot and best by date information around the neck of the unit.

Clover Valley® Instant Coffee was sold and distributed between July 9-21, 2025 exclusively in Dollar General retail stores located in the following states: AL, AR, AZ, CA, CO, CT, DE, FL, GA, IA,ID, IL, IN, KS, KY, LA, MA, MD, ME, MI, MN, MO, MS, MT, NC, ND, NE, NH, NJ, NM, NV, NY, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VA, VT, WA, WI ,WV, and WY.
The recall is being initiated after a customer notified Dollar General employees about the potential issue. Ingesting glass fragments may cause injury to the consumer, and these injuries may include damage to teeth, laceration of the mouth and throat, or perforation of the intestine. No illnesses or injuries have been reported to date.
Customers who purchased this product are encouraged to discard it and contact Dollar General either via email at customercare@dollargeneral.com or by phone at 1-888-309-9030 from 6 a.m. to 1 a.m. CST seven days a week to request a full refund of the purchase price (including any tax).
Dollar General is actively investigating the source of the glass contamination and apologizes for any inconvenience caused by this product issue. The recall is being conducted with the knowledge of the U.S. Food & Drug Administration (FDA).
For additional information, please contact the Media Relations Department at dgpr@dg.com.
About Dollar General Corporation Dollar General Corporation
(NYSE: DG) is proud to serve as America’s neighborhood general store. Founded in 1939, Dollar General lives its mission of Serving Others every day by providing access to affordable products and services for its customers, career opportunities for its employees, and literacy and education support for its hometown communities. As of May 2, 2025, the Company’s 20,582 Dollar General, DG Market, DGX and pOpshelf stores across the United States and Mi Súper Dollar General stores in Mexico provide everyday essentials including food, health and wellness products, cleaning and laundry supplies, self-care and beauty items, and seasonal décor from our high-quality private brands alongside many of the world’s most trusted brands such as Coca Cola, PepsiCo/Frito-Lay, General Mills, Hershey, J.M. Smucker, Kraft, Mars, Nestlé, Procter & Gamble and Unilever.
Hillside Orchard Farms (07/28/2025)
Hillside Orchard Farms is recalling various flavors of their 23oz Fruit Breads & 7.5 oz Fritters due to an undeclared allergen of Egg. People who have an allergy or severe sensitivity to egg run the risk of serious or life-threatening allergic reaction if they consume these products.
On July 17, 2025, during an investigation by the FDA, the firm was made aware that the label failed to include the allergen egg had been left of the label during a reprint of the labels.
No illnesses have been reported to date.
Products affected are:
| PRODUCT | SIZE | LOT/MFG CODES |
USE BY DATE |
|---|---|---|---|
| Apple Bread | 23 oz | 1001 | All dates through 07/23/2025 |
| Peach Bread | 23 oz | 1002 | All dates through 07/23/2025 |
| Strawberry Bread | 23 oz | 1011 | All dates through 07/23/2025 |
| Cinnamon Pecan Bread | 23 oz | 1012 | All dates through 07/23/2025 |
| Blueberry Bread | 23 oz | 1013 | All dates through 07/23/2025 |
| Blackberry Bread | 23 oz | 1014 | All dates through 07/23/2025 |
| Banana Nut Bread | 23 oz | 1016 | All dates through 07/23/2025 |
| Cheese Bread | 23 oz | 1017 | All dates through 07/23/2025 |
| Jalapeno Cheese Bread | 23 oz | 1018 | All dates through 07/23/2025 |
| Apple Fritters | 7.5 oz | 1051 | All dates through 07/23/2025 |
| Peach Fritters | 7.5 oz | 1052 | All dates through 07/23/2025 |
| Strawberry Fritters | 7.5 oz | 1061 | All dates through 07/23/2025 |
| Cinnamon Pecan Fritters | 7.5 oz | 1062 | All dates through 07/23/2025 |
| Blueberry Fritters | 7.5 oz | 1063 | All dates through 07/23/2025 |
| Blackberry Fritters | 7.5 oz | 1064 | All dates through 07/23/2025 |
Recalled products can be identified from the attached photos.
The products were distributed between November 18, 2024 - July 16, 2025. These products were packaged in clear plastic and sold primarily in farm markets and roadside stands located in the States of: Alabama, Georgia, North Carolina, Pennsylvania, and South Carolina.
Consumers who have purchased these products are urged to return them to the place of purchase for a full refund or they may discard the product. Consumers with questions may contact Kiley Mitcham Houston, VP Operations Hillside Orchard Farms at 706-782-4995, Monday to Friday 8:30-5 EST.
This recall is being made with the knowledge of the Food and Drug Administration.













